CARBONATED SOFT DRINK PROCESS
SUGAR SYRUP PREPARATION
Syrup at desired Brix percentage is prepared, please check syrup manufacturing process details in Syrup section
KEY INGREDIENTS IN A TRADITIONAL SOFT DRINK
Ingredients in Traditional Soft Drink
- Water : 90 (for sugar based ) & 98 (for non sugar based) Vol-%
- Sweetners : 8 – 12 % w/v
- Carbon Dioxide : 0.3 to 0.6 % w/v
- Acidulants : 0.05 to 0.3 % w/v
- Flavorings : 0.1 TO 0.5% w/v
- Colors : 0 to 70 ppm
- Preservatives : Class I or II
- Antioxidants : < 100 ppm
- Foaming Agents : E.g : Saponins 200 ppb
- Stabiliser : 0.1 to 0.2 %
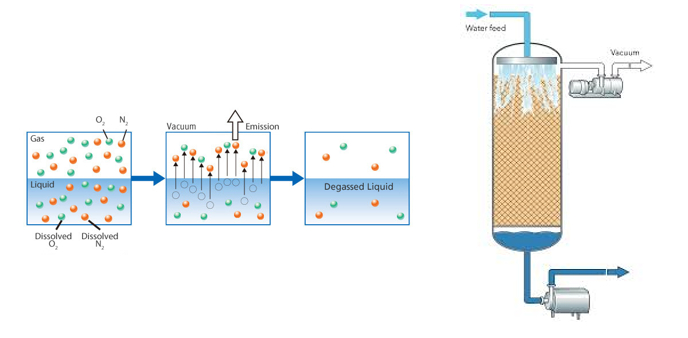
De-aeration of water prior to producing carbonated soft drinks or carbonated water (soda)
The de-aeration of water is an essential part of the carbonation process of most water-based drinks.
Water is normally saturated with air. Whenever we boil some water for cooking, the bubbles we see coming out is not steam but bubbles of air that expand and escape the water.
The water in this case is already saturated with air (which is dissolved in it, the colder the liquid the more gas it will accept before becoming saturated) and as we know when a liquid is saturated with a gas & if we put extra gas in it, the gas shall not dissolve – it shall be rejected.
So, what happens when we open a bottle of carbonated water that has been poorly carbonated without prior de-aeration?
We shall have a flash of CO2 and then the water will go rapidly flat. This is not a great result for our product and the marketing efforts and also for the end customers.
The solution to this situation is that any water that is to be carbonated to produce a soft drink, needs to be treated to first extract the air dissolved in the water before we can carbonate it.
This is done by adding a second column before the carbonating column unit called a DE-AERATOR UNIT and it consists of a column that will extract the air in the water by means of a vacuum pump.
The water is showered down from the top inside a perforated stainless-steel tube, housed inside the main column, in a tube in tube configuration and the vacuum pump, positioned at the top of the de-aerator column takes out the air from the water, which is then sucked out of the column by a secondary pump, and sent to the carbonating unit.
The water is now ready to be mixed with other ingredients and carbonated and chilled to make your soft drink.
There are Two ways of making a SOFT DRINK
Syrup at desired Brix percentage is prepared, please check syrup manufacturing process details in Syrup section
- POST MIX
- PREMIX
What happens in a POST MIX method?
This method is a very popular and traditional method of producing a Soft drink.
Herein, the concentrate is prepared using the above stated key ingredients, this concentrate is then dosed in the containers in a desired proportion which is generated using trial and error method prior to commencement of physical production. Dosing system can be used for precision dosing of concentrates.
These containers are then filled with carbonated water (soda) of desired gas volume, corked / capped and then placed upside down so that the concentrate and soda gets mixed up through the cross stream pressure built up inside the containers.
This method is generally followed by manufacturers with lower volume of production,
Advantages :
- Low cost of set up (Low CAPEX).
- Low machine foot print.
- Easy to operate.
- No skilled labor required.
Disadvantages :
- Taste may vary from batch to batch.
- Hygienic practices are not met.
- High volume production is not possible.
- Loss of gas volume during filling leads to lower soda strength over a period of time.
What happens in a PRE MIX method?
This method is generally used in producing Carbonated soft drink in higher volumes
Herein also we have two modules of manufacturing
a. SEMI AUTO LINES (BATCH LINE)
In this module a blending tank is used to prepare the drink using required proportions of Sugar syrup, purified water and all other key ingredients to form a batch, this drink is then chilled using an appropriate sized chiller & then carbonized using a carbonator at specific gas volume and further sent to counter pressure fillers for Filling in containers.
The drink produced shall remain constant in quality throughout the batch, two tanks are used in this process one for preparing the drink and other for holding the drink, the held up drink is then chilled and used in process of carbonation & in the mean while other batch of the drink is produced.
"Since the process is batched their r chances that the drink may have variations in different batch.
These lines are suitable for low to medium scale production.
b. AUTO LINES (CONTINOUS LINE)
In this module a Premix Automatic PLC controlled beverage memory system is used. The memory can stack different recipes and can operate the system to desired flavors.
The system uses blending based on pre programmed recipe from individual tanks for the key ingredients, blends and carbonates them and sends to filler for filling in containers
This process is a continuous process and is highly precise and is generally used with medium & high-speed lines
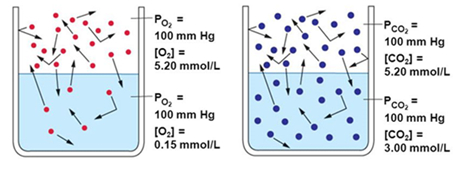
Why only CO2 is used for the process ?
The reason is that carbon dioxide is relatively soluble in water, compared with nitrogen which dissolves only poorly.
At 20 degrees Celsius, Carbon dioxide's solubility in water is 1.688 grams per liter, whereas nitrogen's solubility is 0.019 grams per liter.
It is based on Polar molecules and ions tend to dissolve best in polar solvents.
Since water is a polar substance, it tends to dissolve polar molecules better than it can dissolve nonpolar substances. This is why it can dissolve sugar (polar) and salt (ionic), but not oil (nonpolar). Nitrogen is a nonpolar molecule, so it will not dissolve well in water.
Carbon dioxide is also a nonpolar molecule? but a carbon dioxide molecule can react with a water molecule to form a molecule called carbonic acid.
Carbonic acid, which in solution is actually the carbonate or bicarbonate ion, carries a negative charge which allows it to dissolve in polar liquids like water.
Measuring Carbonisation
Soft drink beverages contain carbonation ranging from 1 to 5 volumes of gas per volume of liquid.
Carbonation is measured as either volumes or grams per litre.
One volume means 1 Litre of CO2 in 1 Litre of drink. This is equivalent to 1.96 g/L (normally quoted as 2 g/L).
A typical carbonated soft drink contains approximately 3–4 volumes (6–8 g/L) CO2. Water at 0oC will dissolve approximately 3.6 g/L CO2.
Typical Carbonization Levels

Counterpressure or Isobaric Filling
A Counter Pressure Filler (also known as an Isobaric Filler) is a device used to fill bottles from a pressurized or non-pressurized bulk storage tank without losing carbonation.
A primary pressurized (or not pressurized) storage tank suitable for carbonated drinks, a chiller and carbon dioxide (CO2) supply storage bottles are all basic tools needed to operate a counter pressure (isobaric) filler. Other required tools are a carbonating unit, this can be combined with the primary storage product tank, or it can be a separate independent carbonating unit.
Description
A counter pressure filler will fill by means of a filling tube from the top of the bottle with a diffuser that distributes the liquid around the walls of the container while filling, to avoid foaming.
The center of the filling tube has a smaller return tube fitted inside that allows the CO2 contained in the pressurized bottle to escape to the top of the filling tank and therefore allowing more product to fill the bottle while the CO2 escapes upwards.
Working principle
A counter pressure (isobaric) bottle filler works by maintaining constant carbon dioxide (CO2) gas pressure on the soft drink as the bottle is filled. In some cases, Bottles and drinks are typically chilled to reduce foaming due to temperature differences.
The bottle is first pressurized with CO2, the fill valve is opened, and the CO2 is then vented to allow the bottle to fill from the bottom.
The process of counter pressure filling consists of the following steps:
- When on the counter-pressure filler the bottle is first sealed by the filling valve gasket.
- Depending on the product to be filled, a vacuum is first created inside the bottle by sucking out the air contained in it.
The filling moment
At this time the bottle is filled by practically only pressurized CO2 at, let’s say, 2.2 to 3.0 bars of pressure.
Gas has been pumped into the bottle to fill it with CO2 and the harmful oxygen is removed
The valve at the top of the filling tank is vented to allow oxygen to escape.
This also pressurizes the bottle to the filling pressure. The CO2 input is then closed.
The filling valve is opened, allowing pre carbonated liquid to flow into the bottle. Pressure is slowly released by the vent allowing CO2 to escape and liquid to flow in and replace CO2 into the bottle.
Because the bottle remains pressurized during the fill, foaming is kept to a minimum. When the bottle is filled, the infeed liquid drink valve is closed.
Remaining pressure is released from the vent and a cap is put on the bottle.
Warming stations
The Filled bottles shall have condensation effect immediately after filling as the filled liquid is chilled.
To remove the water droplets formed :
- for low-speed lines use air knife to blow the droplets from the bottle.
- for high-speed lines use warming tunnels which shall spray warm water over the bottle and bring the temp to normal avoiding condensation and droplet forming.
